Have you ever wondered how scientists can determine the concentration of a colored solution without having to painstakingly count individual molecules? The answer lies in a fundamental principle known as Beer’s Law, which forms the basis of spectrophotometry, a widely used analytical technique in chemistry and beyond.

Image: www.coursehero.com
This article delves into the world of Beer’s Law and its practical application in the laboratory. We’ll explore the concept, its derivation, experimental setup, and various real-world applications. By understanding the intricacies of Beer’s Law, you can gain a deeper appreciation for this powerful tool used to analyze substances and solve scientific problems.
What is Beer’s Law?
Beer’s Law, also known as the Beer-Lambert Law, is a fundamental principle that describes the relationship between the absorbance of light by a solution and the concentration of the absorbing species. It states that the absorbance of a solution is directly proportional to the concentration of the analyte and the path length of the light beam through the solution.
Mathematically, Beer’s Law can be represented as:
A = εbc
Where:
- A is the absorbance of the solution
- ε is the molar absorptivity, a constant that is specific to the absorbing species and the wavelength of light used.
- b is the path length of the light beam through the solution, typically measured in centimeters.
- c is the concentration of the absorbing species, usually expressed in moles per liter (M).
This equation implies that the absorbance of a solution can be used to determine the concentration of the absorbing species, provided that the molar absorptivity and path length are known.
Understanding the Fundamentals
1. The Nature of Light Absorption:
At the heart of Beer’s Law lies the phenomenon of light absorption. When light passes through a solution containing an absorbing species, certain wavelengths of light are absorbed by the molecules of the analyte. The remaining wavelengths are transmitted through the solution, resulting in a change in the light intensity. This change in light intensity is directly proportional to the concentration of the absorbing species in the solution.
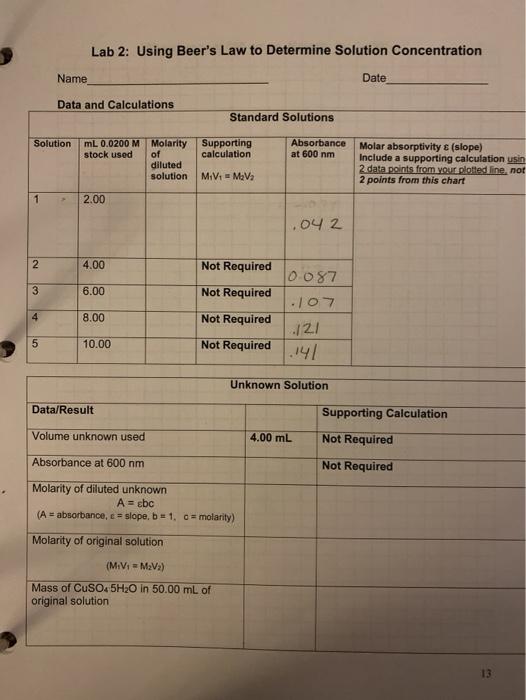
Image: www.chegg.com
2. Molar Absorptivity: A Unique Fingerprint:
Each molecule has a unique ability to absorb light at specific wavelengths. This property is quantified by the molar absorptivity (ε), which represents the absorbance of a 1 M solution of the analyte in a 1 cm path length. The molar absorptivity is a constant that is specific to the analyte and the wavelength of light used. It is a valuable tool for identifying and quantifying different substances based on their unique absorption profiles.
3. Path Length: The Distance Light Travels:
The path length (b) is the distance the light beam travels through the solution. This parameter is critical because the longer the path length, the more opportunities the light has to interact with the absorbing molecules, leading to a higher absorbance. In standard spectrophotometers, the path length is typically 1 cm, ensuring consistent measurements.
Applying Beer’s Law in a Lab Setting: A Practical Guide
Beer’s Law serves as the foundation for spectrophotometry, a widely used technique in chemistry for quantifying the concentration of substances. Here’s a step-by-step guide on performing a Beer’s Law experiment in the lab:
1. Prepare Standard Solutions:
First, prepare a series of standard solutions of the analyte with known concentrations. The range of concentrations should be chosen carefully based on the expected concentration of the unknown sample.
2. Measure Absorbance Using a Spectrophotometer:
A spectrophotometer is an instrument designed to measure the absorbance of light by solutions. In a spectrophotometer, a beam of light is passed through the solution, and the amount of light that is transmitted through is measured. Using Beer’s Law, the absorbance can then be determined.
The spectrophotometer is calibrated using a blank solution, which contains all the components of the sample except the analyte. This ensures that any background absorbance from other components is subtracted from the readings.
3. Plot a Calibration Curve:
Next, plot the absorbance values obtained for each standard solution against their corresponding concentrations. This plot is called a calibration curve. The calibration curve should be linear, indicating that the relationship between absorbance and concentration is directly proportional, as predicted by Beer’s Law.
4. Analyze the Unknown Sample:
Measure the absorbance of the unknown sample using the spectrophotometer. Use the calibration curve to determine the concentration of the analyte in the unknown sample. By finding the absorbance value for the unknown sample on the calibration curve, you can directly read the corresponding concentration.
Applications of Beer’s Law: A Wide Range of Uses
Beer’s Law has numerous applications across various fields, including:
1. Chemistry
- Quantifying chemical reactions: By measuring the absorbance of reactants and products at different time intervals, Beer’s Law can be used to determine the rate and equilibrium constants of chemical reactions.
- Analyzing environmental samples: Beer’s Law is essential for determining the concentrations of pollutants, such as heavy metals and pesticides, in water, soil, and air samples.
2. Biology and Medicine
- Protein and enzyme assays: Beer’s Law is frequently employed to determine the concentration of proteins and enzymes, crucial components in biological systems.
- Drug analysis: Beer’s Law is used to quantify the concentration of drugs in pharmaceutical formulations and biological fluids, ensuring accurate dosage and monitoring drug levels.
- Clinical diagnostics: Spectrophotometry based on Beer’s Law is widely used in clinical laboratories for diagnosing various diseases, including diabetes, anemia, and infectious diseases.
3. Food Science and Technology
- Monitoring food quality: Beer’s Law helps assess the quality of food products by measuring the concentrations of specific components, such as pigments, vitamins, and preservatives.
- Food analysis: Beer’s Law plays a crucial role in determining the composition of food ingredients, ensuring compliance with regulatory standards and maintaining food safety.
Beyond the Beer’s Law Lab Answer Key: A Journey to Deeper Understanding
The Beer’s Law lab answer key provides a crucial framework for understanding and applying the principle in practical settings. But to truly grasp the significance of this fundamental scientific concept, we must embrace a wider understanding of its nuances and limitations.
1. Deviations from Beer’s Law
While Beer’s Law provides a valuable model for understanding light absorption, it’s important to acknowledge that deviations can occur under certain experimental conditions. Factors such as high analyte concentration, intermolecular interactions, and changes in solvent properties can lead to non-linear relationships between absorbance and concentration. Being aware of these deviations is crucial for ensuring accurate results.
2. Importance of Wavelength Selection
Choosing the appropriate wavelength of light for absorbance measurements is crucial for maximizing accuracy. A wavelength at which the analyte exhibits maximum absorbance is typically chosen. This is known as the “lambda max” and allows for the highest sensitivity and precision in concentration measurements.
3. Beyond Spectrophotometry:
The principles of Beer’s Law extend beyond traditional spectrophotometry. Advanced techniques like spectrofluorometry and circular dichroism are based on the same fundamental principles of light absorption but allow for greater sensitivity and provide valuable information about molecular structure and conformation.
Beer’S Law Lab Answer Key Pdf
Conclusion: Unlocking the Secrets of Beer’s Law
Beer’s Law is a cornerstone in analytical chemistry, providing a robust method for quantifying the concentration of substances in solution. This principle, combined with the power of spectrophotometry, underpins a wide range of analytical applications across various fields, from environmental monitoring to drug analysis and food science. By understanding the fundamental principles, exploring its practical applications, and recognizing its limitations, you can unlock the secrets of Beer’s Law and gain a deeper appreciation for the power of this scientific tool.
As you delve further into the world of spectrophotometry and Beer’s Law, remember that the knowledge and skills you acquire through experimentation and analysis will serve as invaluable stepping stones towards a greater understanding of the molecular world. So, continue your scientific journey, embrace the challenges, and unlock the hidden secrets of the universe through the lens of light!






