Have you ever wondered why water is a bent molecule, while carbon dioxide is linear? The answer lies in the realm of molecular geometry, a fascinating field that explores the three-dimensional arrangements of atoms in molecules. One of the most powerful tools for understanding this intricate dance of atoms is the VSEPR theory, which stands for Valence Shell Electron Pair Repulsion. This theory explains how electron pairs around a central atom repel each other, leading to specific molecular shapes that determine a molecule’s properties. Today, we embark on a journey into the heart of VSEPR, exploring its principles and how the Phet simulation can bring these concepts to life.
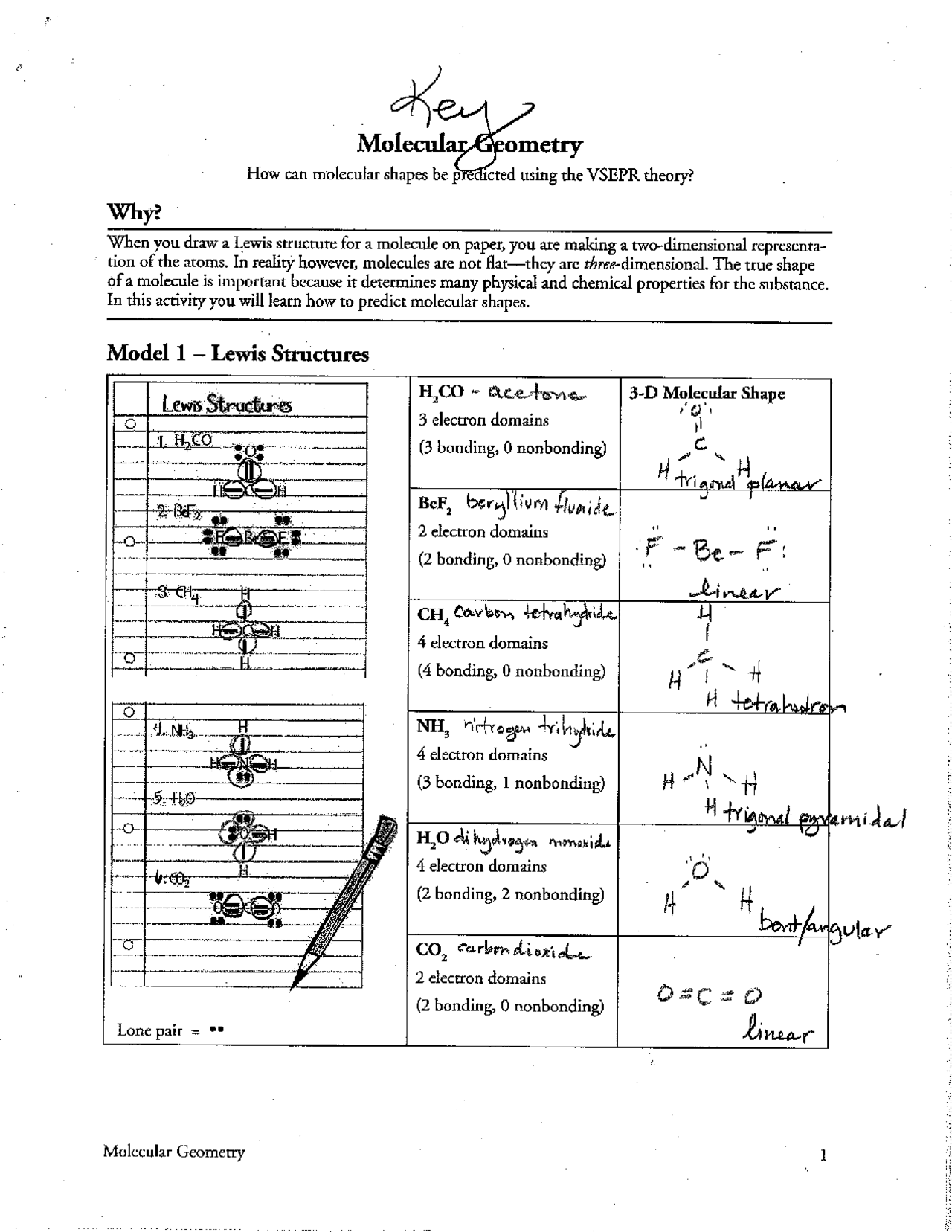
Image: davida.davivienda.com
Imagine a bustling city square, filled with people jostling for space. Each individual seeks to maintain a comfortable distance from others, avoiding collisions. In the microscopic world of molecules, electrons play the role of these individuals, striving to minimize repulsion by arranging themselves in a specific geometric configuration. This is the essence of VSEPR theory, a cornerstone of chemistry that helps predict the shape of molecules based on the arrangement of electron pairs around the central atom. To grasp this fundamental principle, we’ll turn our attention to a valuable tool: the Phet molecular shapes VSEPR activity. This interactive simulation offers a dynamic and engaging way to explore the world of molecular geometry, helping students and enthusiasts alike visualize and understand VSEPR in action.
Delving Deeper into VSEPR: A Journey into Molecular Shapes
VSEPR theory is based on a simple yet profound principle: electron pairs, whether bonding or non-bonding, repel each other. This repulsion leads to specific arrangements of electron pairs around a central atom, determining the overall shape of the molecule. To understand this concept, we need to define some essential terms:
- Lone pairs:These are non-bonding electron pairs, residing on the central atom but not involved in bonding with other atoms. Lone pairs exert a stronger repulsive force than bonding pairs, influencing the molecular shape.
- Bonding pairs: These are shared electron pairs involved in covalent bonds between the central atom and other atoms.
- Electron domains: This refers to the regions around the central atom where electrons are concentrated, encompassing both bonding and lone pairs.
The fundamental tenet of VSEPR theory is that electron pairs arrange themselves to minimize repulsions, leading to specific geometric arrangements around the central atom, summarized in the following table:
| Electron Domains | Geometry | Example |
|---|---|---|
| 2 | Linear | BeCl2 |
| 3 | Trigonal Planar | BF3 |
| 4 | Tetrahedral | CH4 |
| 5 | Trigonal Bipyramidal | PCl5 |
| 6 | Octahedral | SF6 |
The beauty of VSEPR lies in its ability to explain a wide range of molecular shapes, ranging from simple linear molecules to complex octahedral structures. It’s important to remember that the presence of lone pairs can influence the shape of a molecule, leading to deviations from the ideal geometries predicted by VSEPR. For instance, while a molecule with 4 electron domains (like methane, CH4) would ideally have a tetrahedral shape, the presence of lone pairs in ammonia (NH3) distorts this shape, resulting in a trigonal pyramidal geometry.
Embracing the Power of Phet: A Visual Exploration of VSEPR
The Phet molecular shapes VSEPR activity offers a powerful interactive platform for visualizing and understanding VSEPR in action. Through this simulation, you can experiment with different molecules, manipulate their geometries, and observe the effects of lone pairs on molecular shape.
Here’s a step-by-step exploration of the Phet simulation:
- Select a molecule: Begin by choosing a molecule from the list provided, such as water (H2O), ammonia (NH3), or methane (CH4).
- Explore the 3D structure: The simulation allows you to rotate the molecule in three dimensions, providing an immersive view of its structure.
- Examine the electron domains:The simulation highlights the electron domains around the central atom, clearly showing bonding pairs and lone pairs.
- Observe the effect of lone pairs: You can add or remove lone pairs from the central atom, visually observing how the molecular shape changes in response.
- Customize the display: You can change the display settings to show or hide specific elements, such as electron domains, bond lengths, or bond angles.
This interactive exploration fosters a deeper understanding of VSEPR, allowing you to visualize the repulsions between electron pairs and their influence on molecular geometry.
Insights from Experts: Navigating the Complexities of VSEPR
VSEPR theory, while a powerful tool, has its limitations. In some cases, it may not accurately predict the shape of a molecule, especially for more complex molecules with multiple central atoms. In these scenarios, other advanced techniques, such as computational chemistry, may be necessary to obtain a more precise picture of molecular geometry.
However, VSEPR remains a valuable tool for understanding and predicting the shapes of many molecules, laying the foundation for further explorations in chemistry. For those seeking to delve deeper into the intricacies of molecular geometry, consulting authoritative textbooks like “Chemistry: The Central Science” by Theodore L. Brown, H. Eugine LeMay Jr., and Bruce E. Bursten or exploring resources from reputable organizations like the American Chemical Society (ACS) would be highly beneficial.
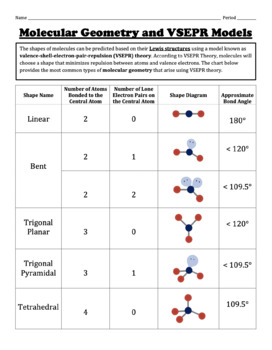
Image: louis-harrington.blogspot.com
Phet Molecular Shapes Vsepr Activity Answer Key Pdf
Unlocking the Secrets of Molecular Shapes: A Call to Action
The world of molecules is full of intricate patterns and fascinating shapes, and VSEPR provides a window into this microscopic realm. By understanding the fundamentals of VSEPR and leveraging the power of interactive simulations like Phet, we can gain valuable insights into the forces that govern molecular structures.
We invite you to explore the Phet molecular shapes VSEPR activity and embark on a journey of discovery, uncovering the fascinating relationships between electron pairs, molecular shapes, and the properties of matter. Share your experiences, thoughts, and any questions you may have in the comments below, fostering a collaborative learning environment for all. Let’s continue to unravel the mysteries of the molecular world, one shape at a time.






