Ever wondered how much sugar is actually in your favorite soda? Or how much salt is present in a packet of instant soup? The answer lies in the intriguing world of concentration, a fundamental concept in chemistry that helps us understand the composition of mixtures and solutions. It’s a practical skill that finds its way into countless aspects of our daily lives, from cooking and baking to understanding the composition of medicines and lab solutions. Today, we’ll embark on a journey to unravel the mysteries of calculating percent by mass and percent by volume, two powerful tools for expressing concentration.
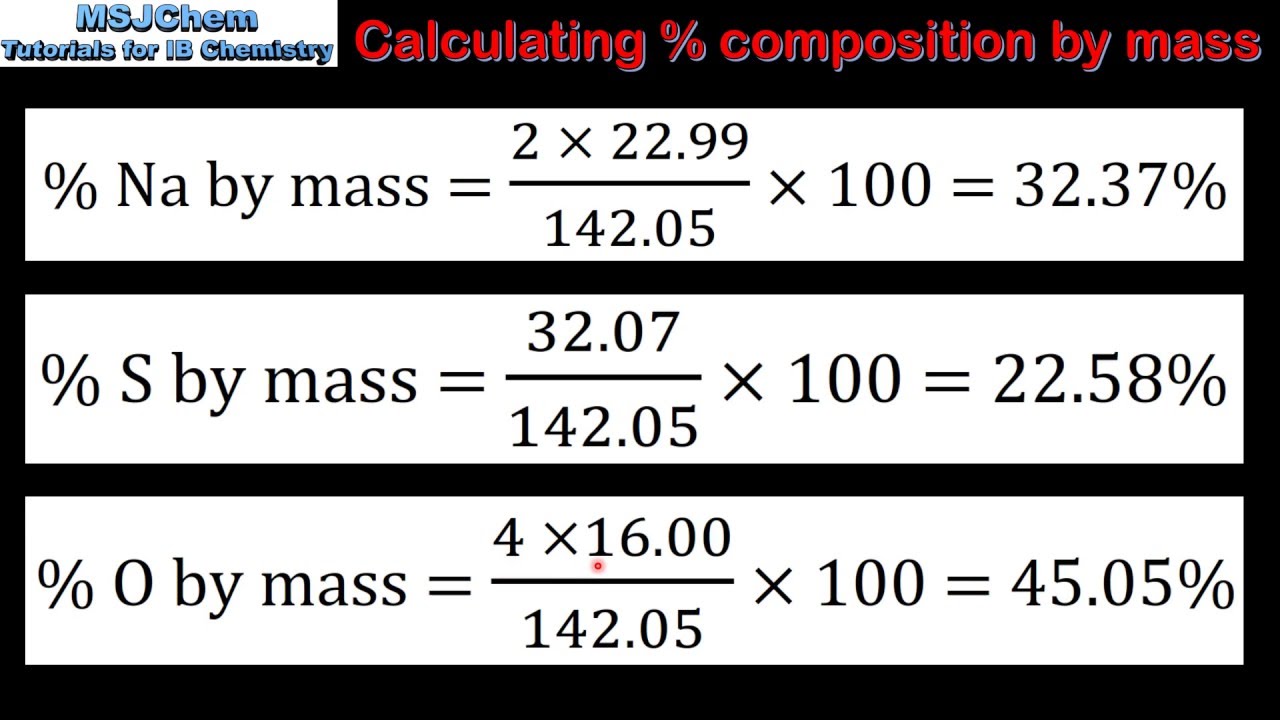
Image: studyfullkristi.z19.web.core.windows.net
This journey will guide you through the basics of percent by mass and percent by volume, providing clear explanations and practical examples to solidify your understanding. We’ll explore the core formulas, delve into real-world applications, and equip you with the knowledge to confidently solve problems related to “calculating percent by mass/volume chem worksheet 15-2.” Ready to unlock the secrets of concentration? Let’s dive in!
Percent by Mass: Unveiling the Composition of Matter
Imagine you’re making a delicious cake. The recipe calls for 200 grams of flour, 100 grams of sugar, 100 grams of butter, and 50 grams of chocolate chips. Knowing the individual masses of each ingredient enables you to understand precisely how much of each component contributes to the final product. This is analogous to understanding the composition of any mixture or solution – knowing the mass of each component allows us to determine its proportion within the whole.
Percent by mass is a way to express the contribution of a specific component, or solute, to the total mass of the mixture or solution. It tells you what percentage of the total mass is made up by that particular component. Mathematically, we can express percent by mass as:
Percent by Mass = (Mass of Solute / Mass of Solution) × 100%
For instance, in our cake batter example, the percent by mass of sugar would be:
Percent by Mass of Sugar = (100 grams Sugar / 450 grams Total Batter) × 100% = 22.22%
This means that 22.22% of the total mass of the cake batter is contributed by sugar.
Percent by Volume: Understanding the Proportions in Liquid Mixtures
Now let’s think about a different situation: making a refreshing fruit punch. You might use one liter of orange juice, 500 milliliters of cranberry juice, and 250 milliliters of ginger ale. Just like with the cake batter, knowing the volumes of each ingredient helps us understand how much of each component contributes to the overall volume. This is where percent by volume comes into play.
Percent by volume expresses the proportion of a specific component (solute) within the total volume of the mixture or solution. In essence, it tells you what percentage of the entire volume is occupied by that specific component. We can express percent by volume mathematically as:

Image: materiallibadler.z4.web.core.windows.net
Percent by Volume = (Volume of Solute / Volume of Solution) × 100%
Applying this to our fruit punch example, the percent by volume of orange juice would be:
Percent by Volume of Orange Juice = (1000 milliliters Orange Juice / 1750 milliliters Total Punch) × 100% = 57.14%
This means that 57.14% of the entire volume of the fruit punch is occupied by orange juice.
Delving Deeper: Real-World Applications of Percent by Mass and Volume
Calculating percent by mass and volume isn’t just confined to baking and beverage creations – it has profound applications in diverse fields:
Chemistry and Medicine:
- Precision in Pharmaceuticals: Determining the percent by mass of active ingredients in medications ensures accurate dosages and effectiveness.
- Balancing Solutions: Scientists use percent by mass and volume to calculate the precise amount of solute needed to create solutions for experiments and research in chemistry labs.
- Intravenous Solutions: Percent by mass and volume play a crucial role in preparing intravenous solutions for medical treatments. The concentration of electrolytes, sugars, and medications needs to be carefully calculated.
Environmental Science:
- Monitoring Pollutants: Percent by mass helps analyze the concentration of pollutants in air, water, and soil to assess environmental health and safety.
- Water Chemistry: Percent by volume can measure the concentration of dissolved oxygen in water bodies, vital for aquatic life.
Industry:
- Industry Quality Control: Manufacturers use percent by mass/volume to ensure consistent product quality and verify that ingredients are present in the required proportions.
- Food Processing: Percent by mass helps determine the amount of ingredients needed for various food products, ensuring consistency in taste, texture, and nutritional value.
Everyday Life:
- Understanding Nutrition Labels: Percent by mass helps us interpret the nutritional information on food labels, clarifying the amount of specific nutrients we consume in our daily diet.
- Mixing Cleaning Solutions: Calculating the percent by volume of ingredients in cleaning solutions ensures effective cleaning without over-dilution or excessive use of harsh chemicals.
Navigating the “Calculating Percent by Mass/Volume Chem Worksheet 15-2”
Now that we’ve established the foundations of percent by mass and percent by volume and explored their vast applications, let’s specifically tackle the “calculating percent by mass/volume chem worksheet 15-2.” While we can’t access the specific problems within the worksheet, we can furnish you with a consolidated approach to tackle any problem involving concentration.
Mastering the Calculation Technique:
- Identify the Solute and Solution: First, understand what component you’re calculating the percentage for (the solute) and what comprises the total mixture or solution.
- Determine the Units: Ensure that the units for the solute and the solution are consistent (e.g., both in grams or milliliters).
- Apply the Formula: Utilize the appropriate formula, either percent by mass or percent by volume, depending on the problem statement.
- Calculate and Express: Substitute the values into the formula, perform the calculation, and express the answer as a percentage.
Additional Tips:
- Double-Check Units: Always verify that your units are consistent to avoid errors in calculations.
- Practice Makes Perfect: Solve multiple problems from different sources and work through various scenarios to solidify your understanding.
- Seek Guidance: Don’t hesitate to ask your teacher, professor, or tutor for clarification on any challenging concepts.
Calculating Percent By Mass/Volume Chem Worksheet 15-2
https://youtube.com/watch?v=P8sCguiq3ko
Conclusion: Concentration – The Key to Understanding Mixtures and Solutions
Exploring the concept of concentration, with a deep dive into calculating percent by mass and percent by volume, reveals its fundamental importance in chemistry and various aspects of our lives. We’ve dissected the concepts, explored real-world applications, and provided a practical approach to tackle problems related to “calculating percent by mass/volume chem worksheet 15-2.” Understanding concentration empowers us to interpret composition, make informed decisions in various fields, and appreciate the interconnectedness of chemistry in our daily lives. So, embrace the knowledge you’ve gained – you’re now equipped to confidently analyze the concentration of solutions and mixtures around you, unlocking the secrets of matter itself!






