Have you ever wondered why the elements on the periodic table have those specific atomic masses? The answer lies in the fascinating world of isotopes. While we often think of an element as having a single, definitive atomic mass, many elements exist in multiple forms, each with a different number of neutrons in their nucleus. These variations are called isotopes, and their proportions in nature – their abundance – play a crucial role in understanding the chemistry of the world around us.

Image: lessonlibrarymoss55.z19.web.core.windows.net
This article delves into the concept of isotopic abundance, exploring its importance in various fields, including chemistry, geology, and even archaeology. We’ll examine how scientists determine the abundances of different isotopes, how these values are used in applications ranging from dating ancient artifacts to tracking environmental pollution, and how Chem Worksheet 4.3 provides a valuable tool for learning about this intriguing subject.
Unveiling the Isotopic Landscape: Understanding the Basics
Imagine a family portrait: each member shares common features, but they also have unique characteristics that set them apart. Similarly, isotopes of an element share the same number of protons (defining the element), but they differ in the number of neutrons in their nucleus. For example, carbon (C) exists as two major isotopes: Carbon-12 (12C) with 6 protons and 6 neutrons and Carbon-14 (14C) with 6 protons and 8 neutrons. These differences in neutrons lead to slightly varying atomic masses, while maintaining the chemical properties that define the element.
The relative abundance of isotopes of an element is crucial in chemistry. It’s not just about averages; it’s about the proportions of each isotope within a sample. These proportions directly affect the element’s average atomic mass, which is a weighted average of the masses of its isotopes based on their relative abundances. This average atomic mass is what you see on the periodic table.
Dissecting Isotopic Abundance: Methods of Determination
Scientists use various techniques to determine the abundance of isotopes. One primary method is mass spectrometry. This powerful technique separates ions of different masses in a magnetic field. The abundance of each isotope can be calculated from the intensity of its signal in the spectrometer, providing a precise measurement of isotopic composition.
Another technique, nuclear magnetic resonance (NMR) spectroscopy, utilizes the magnetic properties of atomic nuclei to differentiate between isotopes. By analyzing the specific signals produced by different isotopes, NMR spectroscopy can provide insights into isotopic abundance. This method is particularly useful in studying the distribution and chemical environment of different isotopes within molecules and materials.
Isotopes: Beyond the Textbook: Applications in the Real World
The concept of isotopic abundance isn’t confined to the pages of textbooks; it plays a vital role in diverse real-world applications. Let’s explore some of its key applications:
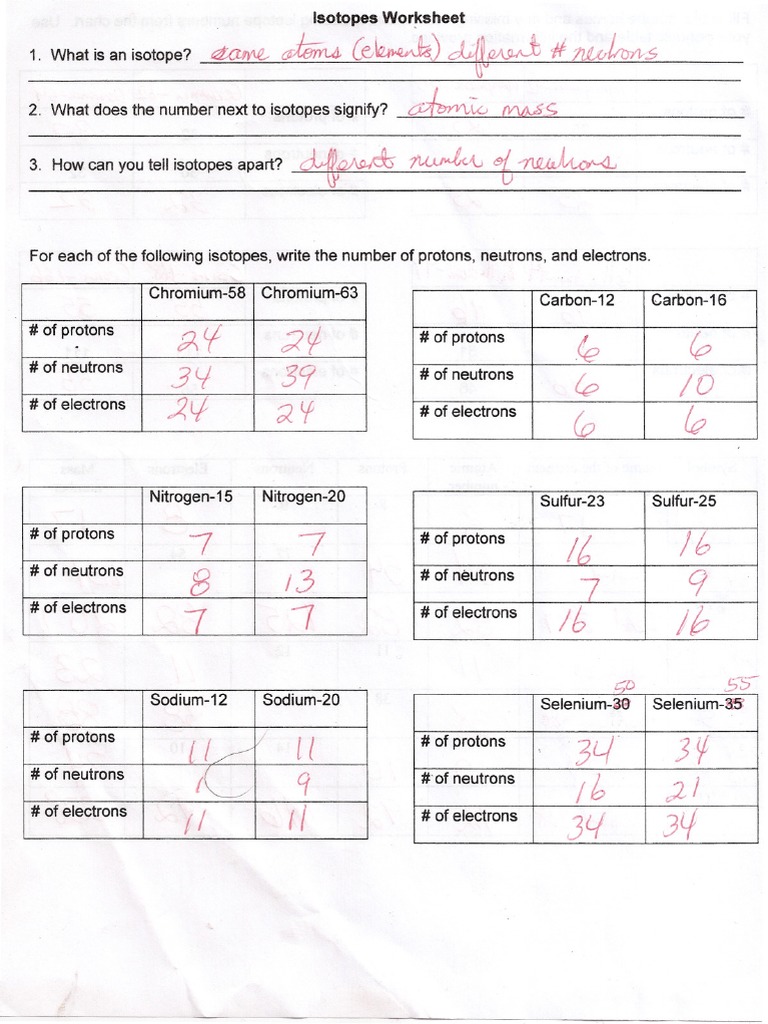
Image: learninglibraryrosado.z13.web.core.windows.net
1. Dating Ancient Artifacts: Unveiling the Past
Carbon-14 dating, a cornerstone of archaeology, relies on the radioactive decay of Carbon-14, an isotope with a half-life of approximately 5,730 years. This method allows scientists to determine the age of ancient artifacts, fossils, and even the age of Earth itself. The relative abundance of Carbon-14 in a sample compared to Carbon-12 reveals its time of origin, unraveling secrets from the distant past.
2. Tracking Environmental Pollution: Mapping Contamination
Isotopic analysis is employed to track the origin and movement of pollutants in the environment. For instance, the analysis of lead isotopes in soil and water samples helps scientists trace the source of lead contamination, whether it be from industrial waste or natural deposits. This data is critical in developing strategies for environmental remediation.
3. Understanding Geological Processes: Deciphering Earth’s History
Geologists use isotopic signatures to understand the formation and evolution of our planet. For example, the different ratios of isotopes in rocks can shed light on the age of earth formations, volcanic eruptions, and tectonic plate movement. This information is fundamental to comprehending the dynamics of Earth’s geological processes.
4. Medical Imaging and Diagnosis: A Window into the Body
Isotopes are used in medical imaging and diagnosis. Technetium-99m, a radioactive isotope with a short half-life, is commonly used in bone scans, allowing doctors to visualize bone structures and detect abnormalities. Other isotopes, such as iodine-131, are employed in thyroid scans to assess the function of the thyroid gland.
Chem Worksheet 4.3: Navigating the World of Isotopes
Chem Worksheet 4.3 provides a valuable resource for understanding the concept of isotopic abundance in a comprehensive and engaging manner. The worksheet typically includes:
- Definitions and Concepts: A clear explanation of isotopes, isotopic abundance, average atomic mass, and related concepts.
- Practice Problems: A variety of practice problems designed to test understanding of concepts and calculations involving isotopic abundance.
- Real-world Examples: Application-based exercises that demonstrate the significance of isotopes in various fields, such as archaeology, geology, and medicine.
By working through Chem Worksheet 4.3, students can develop a solid foundation in isotopic abundance and appreciate its relevance in the scientific world.
Abundance Of Isotopes Chem Worksheet 4 3
Conclusion: Isotopic Abundance: A Cornerstone of Chemistry
The concept of isotopic abundance is a testament to the intricate nature of chemistry and the diverse ways in which elements behave. This seemingly abstract concept has practical implications, touching on numerous fields, from archaeology and geology to medicine and environmental science. By understanding how isotopes vary in their abundance, we gain a deeper appreciation for the world around us and the powerful tools used to explore it.
So, the next time you encounter an element on the periodic table, remember that it’s not just a singular entity but represents a family of isotopes, each with its unique abundance and contribution to the story of our planet. Chem Worksheet 4.3 serves as an excellent stepping stone for unraveling this fascinating world of isotopes and appreciating their remarkable role in shaping our universe.






