Remember those childhood chemistry sets where you mixed different powders and watched them fizz and change color? That was our first glimpse into the fascinating world of chemical reactions. At its core, the way atoms interact with one another is governed by their desire to achieve stability, and sometimes, that involves the exchange of electrons in a process known as ionic bonding. Understanding ionic bonds is vital for comprehending how molecules form and the properties of the compounds they make up. The Student Exploration Gizmo on Ionic Bonds offers an engaging way to visualize and explore this fundamental concept.

Image: athensmutualaid.net
For many of us, visualizing abstract concepts like atomic structure and electron transfer can be tricky. This is where the Student Exploration Gizmo comes in. This interactive tool provides a simulated environment where you can manipulate atoms, observe their electron configurations, and witness the dramatic formation of ionic bonds. By using the Gizmo, you can explore a specific ionic bond like the one between sodium and chlorine and understand how the transfer of electrons contributes to the bond’s formation and the resulting properties of the compound.
Delving Deeper into Ionic Bonds: A Journey into the World of Chemistry
Defining Ionic Bonds: The Chemistry of Attraction and Repulsion
An ionic bond is a type of chemical bond that forms between two atoms due to the electrostatic attraction between oppositely charged ions. One atom, typically a metal, loses electrons to become a positively charged ion called a *cation*. The other atom, usually a nonmetal, gains electrons to become a negatively charged ion called an *anion*. These oppositely charged ions are then drawn together by the compelling force of electrostatic attraction, forming the ionic bond.
How Ionic Bonds Work: Unveiling the Chemistry of Electron Transfers
The driving force behind ionic bond formation is the pursuit of stability. Atoms seek to achieve a stable, filled outer shell of electrons. Elements like sodium (Na), with one electron in their outermost shell, readily lose that electron to achieve a stable configuration. Chlorine (Cl), on the other hand, has seven electrons in its outermost shell, and it readily gains an electron to achieve a stable eight-electron configuration.
When sodium and chlorine interact, sodium will transfer its single outer electron to chlorine, creating a positively charged sodium ion (Na+) and a negatively charged chloride ion (Cl-). These ions are then electrostatically attracted to each other, forming an ionic bond and creating the compound sodium chloride (NaCl), commonly known as table salt.
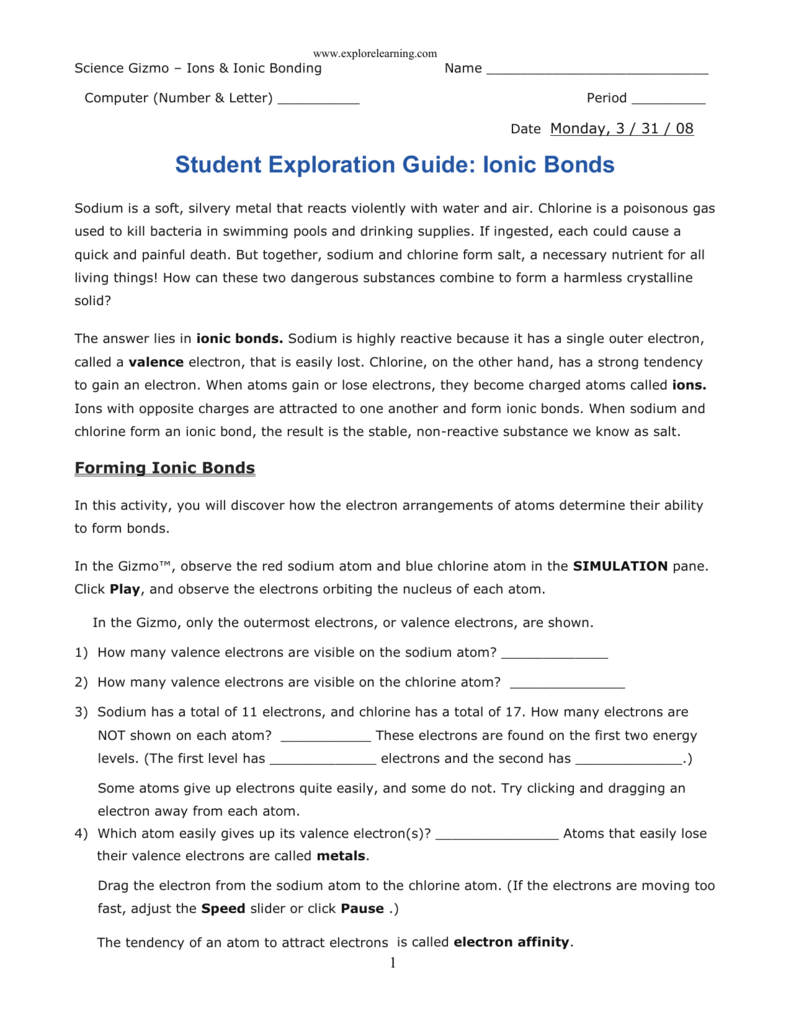
Image: yachestefany.blogspot.com
Properties of Ionic Compounds: A Look at the Outcome of Ionic Bonding
The resulting ionic compounds exhibit distinct properties that differ significantly from their parent atoms. They typically form crystalline solids with high melting and boiling points. This is due to the strong electrostatic forces holding the ions together in a tightly packed lattice structure. Ionic compounds are also generally good conductors of electricity when dissolved in water or molten. This conductivity arises from the ions being able to move freely in the liquid state, enabling the flow of electric current.
Examples of Ionic Compounds: Unveiling the Prevalence of Ionic Bonds in Nature
Ionic bonds are pervasive in nature. They play vital roles in countless chemical reactions and the formation of various compounds with diverse functionalities. Here are some common examples:
- Sodium chloride (NaCl): The ubiquitous table salt used in cooking and food preservation.
- Calcium carbonate (CaCO3): The primary component of seashells, limestone, and marble.
- Potassium chloride (KCl): Essential for electrolyte balance in the human body.
- Magnesium oxide (MgO): Used in various applications, including refractories and insulation.
Ionic Bond Formation: The Role of the Student Exploration Gizmo
The Student Exploration Gizmo on Ionic Bonds provides a visually engaging and interactive way to grasp the concept of ionic bond formation. You can use this tool to experiment with different atoms, observe the electron transfer process, and witness the formation of ionic compounds.
Unlocking the Secrets of Ionic Bonds: Tips and Expert Advice
To make the most of your exploration and achieve a deeper understanding of ionic bonds, consider these tips:
- Focus on electron configurations: Start by understanding the electron configurations of the participating atoms. This will help you visualize the transfer of electrons and the formation of ions.
- Explore different ionic bonds: Don’t limit your exploration to sodium chloride alone. Experiment with various combinations of metals and nonmetals to observe the diversity of ionic bonds.
- Relate properties to structure: As you observe the formation of ionic compounds, note the properties of the resulting compounds. Connect these properties to the structure of the ionic lattice.
- Draw diagrams: Create your own diagrams of ionic bond formation to solidify your understanding of the process. This can be a great way to visualize electron transfer and ionic attractions.
By actively engaging with the Gizmo and following these tips, you can achieve a comprehensive understanding of ionic bonds and their critical role in chemistry.
FAQs: Addressing Common Questions about Ionic Bonds
Q: Can ionic bonds form between only two atoms?
A: Yes, ionic bonds can form between two atoms, such as in the case of sodium chloride (NaCl). However, ionic compounds often consist of a larger number of atoms arranged in a crystalline lattice structure.
Q: Why are ionic compounds typically solids at room temperature?
A: The strong electrostatic forces holding the ions together in a tightly packed lattice structure result in high melting and boiling points. Consequently, ionic compounds are typically solid at room temperature.
Q: How are ionic bonds different from covalent bonds?
A: Both ionic and covalent bonds involve the sharing of electrons, but the extent of sharing differs. In ionic bonds, electrons are transferred completely from one atom to another, resulting in the formation of ions with opposite charges. In covalent bonds, electrons are shared between atoms to achieve stability.
Q: What is the role of electronegativity in ionic bonding?
A: Electronegativity is a measure of an atom’s tendency to attract electrons. In ionic bonding, the atom with a higher electronegativity will gain electrons, becoming an anion, while the atom with a lower electronegativity will lose electrons, becoming a cation.
Q: Why are ionic compounds good conductors of electricity when dissolved in water?
A: When ionic compounds dissolve in water, the ions are free to move throughout the solution. This mobility allows the ions to carry electrical charges, making the solution conductive.
Student Exploration Gizmo Answers Ionic Bonds
Conclusion: Expanding Your Knowledge of Chemical Bonding
Understanding ionic bonds is crucial for gaining a deeper appreciation for the intricate world of chemistry. By using the Student Exploration Gizmo and following the tips provided, you can gain a solid foundation in this fundamental concept. Remember to explore different combinations of atoms and observe the resulting compounds. The more you practice and visualize, the better your grasp of ionic bonding will become.
Do you find the exploration of ionic bonds through the Student Exploration Gizmo to be an engaging learning experience? Share your thoughts and experiences in the comments below.






