Have you ever wondered how molecules stick together, forming the building blocks of everything around us? From the air we breathe to the water we drink, the intricate dance of atoms within molecules is governed by the fascinating world of chemical bonding. This invisible force, holding atoms in a tight embrace, dictates the properties of everything from the shimmering diamond to the humble salt we sprinkle on our food. Today, we embark on an exploration of two fundamental types of chemical bonding: ionic bonding and covalent bonding.
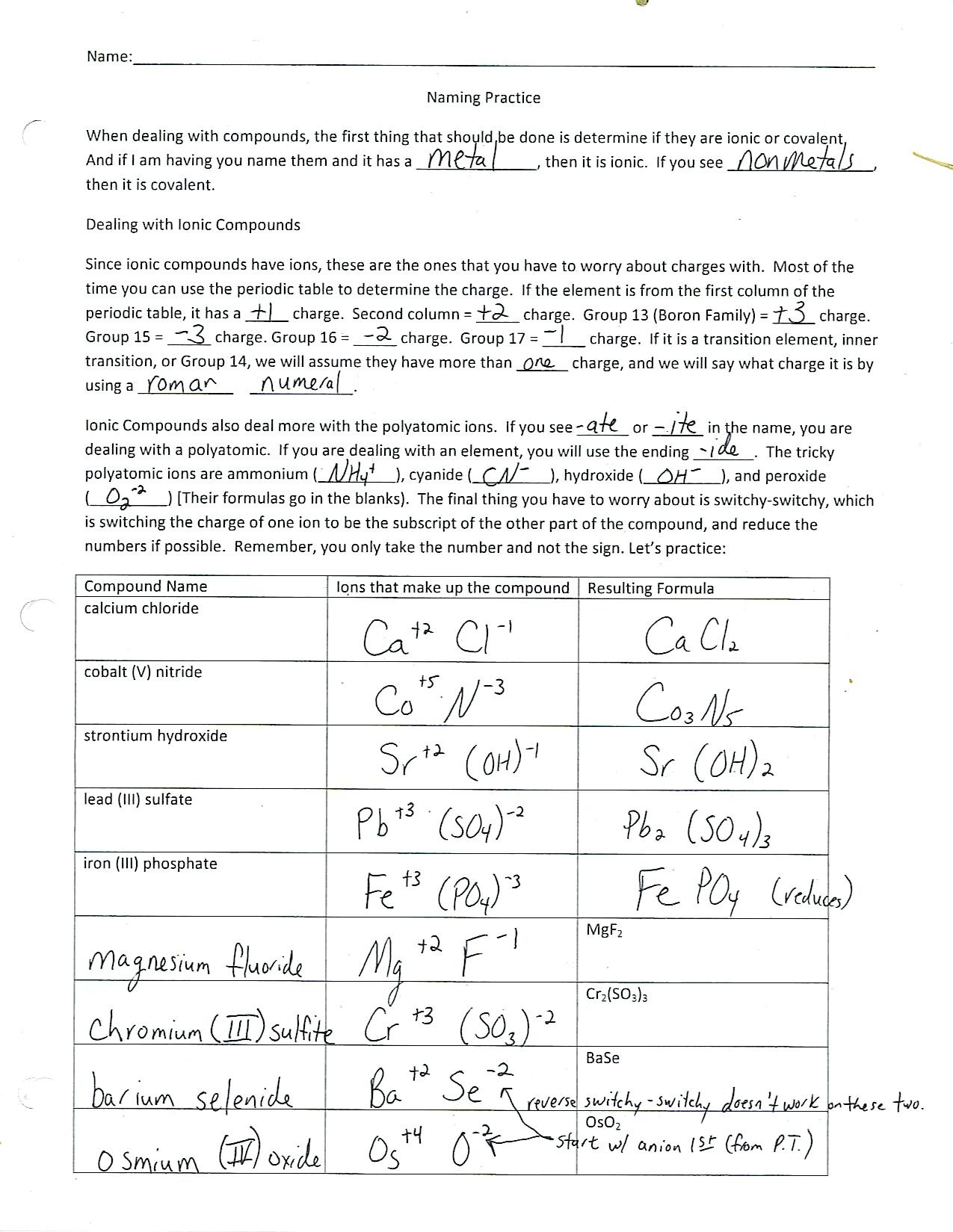
Image: worksheetlibrarycopp.z13.web.core.windows.net
Understanding chemical bonding is crucial, not just for academic pursuits but for grasping the very essence of the world around us. By understanding these bonds, we can unravel the mysteries behind the behavior of matter, from the formation of water droplets to the powerful reactions taking place in our bodies. Let’s begin by diving into the core concepts of ionic and covalent bonds, their unique characteristics, and the fascinating ways they dictate the properties of the substances they form.
The Art of Ionic Bonding: A Transfer of Love
Imagine a love story where one partner, generous and giving, bestows its electron upon the other, creating a bond forged by electrostatic attraction. This is the essence of ionic bonding, a captivating dance between atoms with drastically different personalities—the electron-hungry nonmetals and the electron-donating metals.
The Dance of Electrons
The story begins with metals, readily giving up their electrons, becoming positively charged ions. Nonmetals, on the other hand, eagerly accept these electrons, transforming into negatively charged ions. These oppositely charged ions, like magnets drawn to their poles, unite, forming stable compounds. The electrostatic attraction between these charged ions forms the bedrock of ionic bonding.
Examples of Ionic Bonding
Let’s take a peek into the real world of ionic bonding:
- Sodium Chloride (NaCl): The familiar table salt we use every day is a stunning example. Sodium (Na), a metal, happily donates its electron to chlorine (Cl), a nonmetal. The resulting positive sodium ion (Na+) and negative chloride ion (Cl-) are held together by the irresistible force of electrostatic attraction, forming a crystal lattice of sodium chloride.
- Magnesium Oxide (MgO): Magnesium (Mg), a generous metal, sheds two electrons, becoming a positively charged ion (Mg2+). Oxygen (O), a nonmetal with a strong appetite for electrons, accepts these two electrons, transforming into a negatively charged ion (O2-). The electrostatic attraction between these oppositely charged ions forms magnesium oxide, a compound vital for various industrial applications.

Image: answerlibraryshyla88.z13.web.core.windows.net
Properties of Ionic Compounds
The love story of ionic bonds gives rise to a unique set of properties:
- High Melting and Boiling points: Ionic bonds, due to the strong electrostatic attraction, require a significant amount of energy to break apart, leading to high melting and boiling points.
- Solid State at Room Temperature: The rigid structure of ionic compounds, with ions tightly packed in crystal lattices, results in their solid state at room temperature.
- Conductivity in Molten or Aqueous State: While ionic solids are poor conductors of electricity, when dissolved in water or melted, the ions become mobile, allowing them to conduct electricity.
- Brittle Nature: When stressed, the rigid structure of ionic compounds can easily cleave, leading to their brittle nature.
The Sharing of Love: Covalent Bonding
In a world of equal love and sharing, atoms come together, blending their electrons to achieve a state of harmony. This is the core of covalent bonding, a symphony of shared electrons, where nonmetals share electrons to achieve a stable electron configuration.
Shared Electrons, Shared Happiness
Nonmetals, with their inherent love for electrons, find solace in sharing their electrons with other nonmetals. Each shared electron orbit both participating atoms, forming a strong bond built on mutual satisfaction.
Types of Covalent Bonds
Covalent bonds come in various forms, each with its unique flavor:
- Single Covalent Bond: The sharing of one electron pair between two atoms is the foundation of a single covalent bond, as exemplified by the bond in the hydrogen molecule (H2).
- Double Covalent Bond: Sharing two electron pairs yields a double covalent bond, as seen in the bond between carbon atoms in ethylene (C2H4).
- Triple Covalent Bond: Sharing three electron pairs results in a triple covalent bond, as observed in the bond between nitrogen atoms in nitrogen gas (N2).
Examples of Covalent Bonding
Let’s explore the dance of shared electrons in the real world:
- Water (H2O): Each hydrogen atom shares an electron with the oxygen atom, forming two single covalent bonds, resulting in the familiar water molecule.
- Carbon Dioxide (CO2): Carbon, with its four electrons to share, forms a double covalent bond with each oxygen atom, yielding carbon dioxide, a gas crucial for life.
Properties of Covalent Compounds
The shared love story of covalent bonds leads to a distinct set of properties:
- Lower Melting and Boiling points: Compared to ionic compounds, covalent compounds generally have lower melting and boiling points due to the weaker intermolecular forces arising from the sharing of electrons.
- Gaseous, Liquid, or Solid at Room Temperature: The weaker intermolecular forces in covalent compounds allow them to exist in various states at room temperature, from gases like oxygen to liquids like water to solids like sugar.
- Poor Conductivity of Electricity: The absence of free mobile charges in covalent compounds generally makes them poor conductors of electricity.
Bridging the Gap: Polar Covalent Bonds
While some relationships are perfectly balanced, others involve a tug-of-war, where one partner attracts the shared electrons more strongly than the other. This uneven sharing of electrons, present in polar covalent bonds, introduces a new dimension to the tale of chemical bonding.
Uneven Sharing: A Tale of Electric Dipole
In polar covalent bonds, one atom, often a highly electronegative atom like oxygen, exerts a stronger pull on the shared electrons, creating a partial positive charge on the less electronegative atom and a partial negative charge on the more electronegative atom. This uneven distribution of charge results in a polar molecule, resembling a tiny magnet with positive and negative poles.
Examples of Polar Covalent Bonding
Water (H2O) is a prime example of a polar covalent molecule. Oxygen, with its strong electronegativity, attracts the shared electrons more strongly, creating a partial negative charge on the oxygen atom and partial positive charges on the hydrogen atoms. This polarity plays a vital role in water’s unique properties, such as its high boiling point and its ability to dissolve many ionic compounds.
The Interplay of Forces: Intermolecular Forces
Beyond the realm of atoms, we encounter another force that shapes the behavior of molecules: intermolecular forces. These forces, weaker than the covalent and ionic bonds that hold atoms together, act between molecules, influencing their attraction, properties, and interactions.
There are various types of intermolecular forces, each with its own strength and influence:
- Hydrogen Bonding: A strong type of intermolecular force involving a hydrogen atom linked to a highly electronegative atom like oxygen, nitrogen, or fluorine. This force plays a crucial role in the properties of water and biological molecules.
- Dipole-Dipole Interactions: Attractions between polar molecules, arising from the electrostatic interactions between the positive end of one molecule and the negative end of another. These interactions contribute to the properties of polar liquids.
- London Dispersion Forces: Weakest intermolecular forces, present in all molecules, arise from temporary fluctuations in electron distribution. While weak individually, they become significant in nonpolar molecules and at low temperatures.
Chemical Bonding: A Framework for the World
The remarkable tapestry of chemical bonding, with its diverse forms and intricate forces, governs the properties and interactions of matter. By understanding these bonds and their interplay, we gain insights into the behavior of various substances, from the formation of compounds to the processes of life itself.
Applications of Chemical Bonding
The knowledge of chemical bonding is integral to numerous fields:
- Chemistry: Predicting chemical reactions, understanding the formation of molecules, and synthesizing new materials.
- Biology: Understanding the structure and function of biomolecules like proteins, DNA, and carbohydrates.
- Medicine: Developing new drugs and understanding how existing medications interact with the body.
- Materials Science: Engineering materials with specific properties, such as strength, conductivity, and heat resistance.
Worksheet Chemical Bonding – Ionic & Covalent
The Journey Continues…
Our exploration of chemical bonding has just begun. This vast and captivating field offers countless opportunities for further investigation. From the intricate details of bond formation to the diverse applications of chemical bonding, there is an abundance of knowledge waiting to be discovered, unlocking the mysteries of the world around us. Embrace the wonders of chemical bonding, continue your exploration, and let the beauty of these fundamental forces inspire awe and wonder!






